The gas sensor and its measuring principles
The gas sensor - the mainpiece of our gas measuring systems
Our gas measuring systems are equipped with a gas sensor and a transmitter. The gas sensor detects the concentration of a gas or steam in atmospheric air, the transmitter prepares the sensor signals and outputs them as analog or digital signals.
Electrochemical measuring principle (EC sensor)

Electrochemical gas sensors (also: electrochemical cells, EC sensors) function similarly to batteries.
The gas diffuses into the sensor and is measured at the measuring electrode either:
oxidized (e.g., CO + H2O = CO2 + 2H+ + 2e- )
or reduced (e.g.,O2 + 2 H2O + 4 e- = 4OH- ).
The resulting ions (H + or OH-) diffuse through the liquid electrolyte and are deposited on the counter electrode either:
reduced (e.g.,O2 + 4H+ + 4e- = 2H2O)
or oxidized (e.g., 4OH- + Pb = PbO2 + 2H2O + 4e-).
Between the two electrodes, electricity flows proportional to the gas concentration.
A reference electrode generates a constant potential and can thus prevent drifting of the measured values.
Electrochemical gas sensors are usually specific, there is little or no cross-sensitivity to other substances.
Dynamic zirconia dioxide sensor for O2
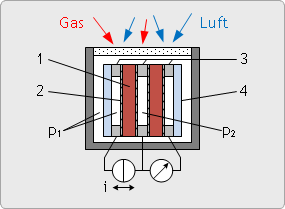
Our oxygen measuring systems contain as its centerpiece a dynamic oxygen sensor based on zirconium dioxide and can measure fail-safe as required.
Read how the dynamic oxygen sensor works, how to detect faults and what partial pressure means in the article "Oxygen measurement" of our knowledge base.
to the article oxygen measurement
Two-beam infrared sensor (NDIR) for CO2
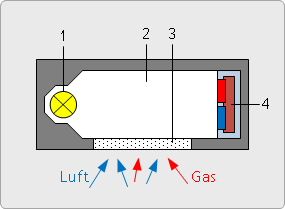
This gas sensor is a company-owned development. It is specific to CO2.
Read how CO2 measurement with our NDIR sensor works in the article "Carbon Dioxide Measurement" of our knowledge base.
To the article Carbon dioxide measurement
Semiconductor gas sensor
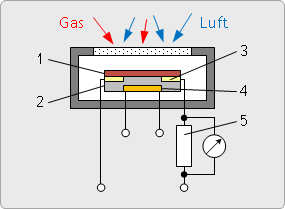
A metal oxide based (SnO2) semiconductor is deposited on a substrate. The substrate includes electrodes that measure the resistance of the semiconductor, and a heater that heats the semiconductor from 200 °C up to 400 °C.
The sensor responds to changes in the composition of the ambient atmosphere with a change in the resistance of the semiconductor.
Reducing gases such as, for example, CO or H2 reduce the resistance of the semiconductor.
The sensitivity of the semiconductor for a particular gas can be varied over the temperature of the semiconductor.
Catalytic gas sensor (pellistor, heat sensor)
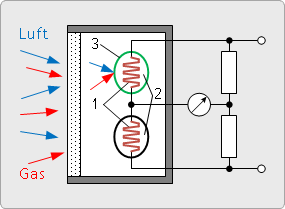
Two platinum filaments are each embedded in a ceramic layer and electrically connected via a bridge circuit. The surface of one platinum helix is activated with a catalyst which promotes oxidation, the surface of the other platinum helix is inactivated.
Electricity flows through the coils and heats them up to about 500°C.
On the surface of the active filament, the oxygen reacts with the combustible gas.
This increases the temperature and resistance in the active platinum helix.
The bridge becomes imbalanced. This can be measured.


